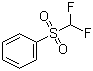
I-Difluoromethyl phenyl sulfone (CAS# 1535-65-5)
Umngcipheko kunye noKhuseleko
| Iimpawu ezinobungozi | Xi – Irritan |
| Iikhowudi zoMngcipheko | I-36/38-Icaphukisa amehlo kunye nolusu. |
| Inkcazelo yoKhuseleko | S26 – Xa udibana namehlo, hlambulula ngokukhawuleza ngamanzi amaninzi kwaye ufune iingcebiso zonyango. S24/25-Kuphephe ukudibana nolusu namehlo. |
| WGK eJamani | 3 |
| I-TSCA | No |
| Ikhowudi ye-HS | 29309090 |
Intshayelelo
I-Difluoromethylbenzenyl sulfone yikhompawundi ephilayo. Nazi ezinye zeempawu zayo:
1. Imbonakalo: I-Difluoromethylbenzenyl sulfone ayinambala ukuya kwikristale ekhanyayo emthubi okanye umgubo.
4. Uxinaniso: Inoxinano malunga ne-1.49 g/cm³.
5. I-Solubility: I-Difluoromethylbenzosulfone i-soluble kwezinye i-solvents eziphilayo, ezifana ne-ethanol, i-dimethyl sulfoxide kunye ne-chloroform. Inonyibiliko oluphantsi emanzini.
6. Iimpawu zemichiza: I-Difluoromethylbenzenylsulfone yikhompawundi ye-organosulfur, enokuthi ifumane iimpendulo ze-organic sulfure, ezifana ne-nucleophilic substitution reaction kunye ne-electrophilic substitution reaction. Isenokusetyenziswa njengomnikeli weeathom zefluorine kwaye inendima ekhethekileyo kwezinye iintshukumo ze-organic synthesis.
Akuvumelekanga ngokungqongqo ukuba udibane nezinto ezinamandla ze-oxidizing ezifana ne-oxidants ukuphepha ingozi. Ukusetyenziswa ngokufanelekileyo kunye nokugcinwa kwe-difluoromethylphenylsulfone kubaluleke kakhulu.