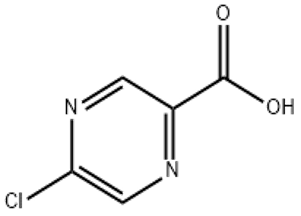
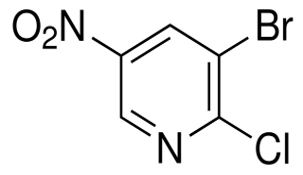
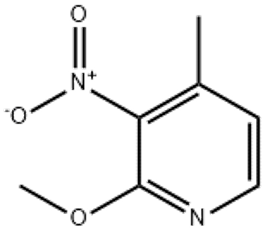
Irisone(CAS#14901-07-6)
| Iikhowudi zoMngcipheko | I-R42/43 – Inokubangela uvakalelo ngokuphefumla kunye nokudibana nolusu. |
| Inkcazelo yoKhuseleko | S24/25-Kuphephe ukudibana nolusu namehlo. |
| WGK eJamani | 2 |
| RTECS | EN0525000 |
| I-TSCA | Ewe |
| Ikhowudi ye-HS | 29142300 |
ukwazisa
indalo
IViolet ketone, ekwabizwa ngokuba yi-linaylketone, yinxalenye yendalo yeketone. Iyona nxalenye ephambili yephunga leentyantyambo ze-violet.
IViolet ketone lulwelo olunamafutha angenambala ukuya kumthubi noluguquguqukayo kwiqondo lobushushu begumbi.
IViolet ketone iyanyibilika etywaleni nakwizinyibilikisi ze-ether, kwaye inyibilika kancinane emanzini. Ubuninzi bayo buphantsi, kunye nobuninzi be-0.87 g/cm ³. Inochuku ekukhanyeni kwaye inokufunxa imitha ye-ultraviolet.
I-Violet ketone inokuthi i-oxidized kwi-ketone alcohols okanye i-acids kwi-chemical reactions, kwaye inokwehliswa kwi-alcohols ngokusebenzisa iimpendulo zokunciphisa i-hydrogenation. Inokungena kwi-alkylation kunye ne-esterification reactions kunye neekhompawundi ezininzi.
Ukusetyenziswa kunye nendlela yokudibanisa
IViolet ketone (ekwaziwa njenge ketone emfusa) yinkompawundi yeketone enuka kamnandi. Inevumba elikhethekileyo kwaye isoloko isetyenziswa kushishino lweziqholo neziqholo. Oku kulandelayo yintshayelelo yokusetyenziswa kunye neendlela zokudibanisa ionone:
Injongo:
I-Perfume kunye ne-spice: iimpawu zevumba le-ionone, esetyenziswa kakhulu kwishishini le-perfume kunye neziqholo ukwenza iimveliso zevumba elibomvu.
Indlela yokudibanisa:
Udibaniso lwe-ionone luphunyezwa ngokubanzi ngezi ndlela zimbini zilandelayo:
I-Oxidation ye-Nucleobenzene: I-Nucleobenzene (iringi ye-benzene ene-methyl substituent) iphantsi kwe-action ye-oxidation, efana nokusebenzisa i-asidi ene-oxidizing okanye isisombululo esineasidi se-potassium permanganate, ukwenza i-ionone.
Ukudityaniswa kwe-Pyrylbenzaldehyde: I-Pyrylbenzaldehyde (efana ne-benzaldehyde kunye ne-pyridine ring substituents kwi-para okanye kwi-meta position) iphendulwa nge-acetic anhydride kunye nezinye i-reactants phantsi kweemeko ze-alkaline ukwenza i-ionone.